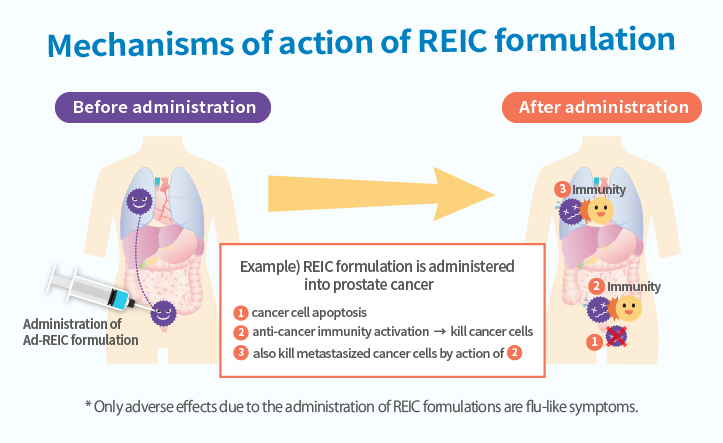
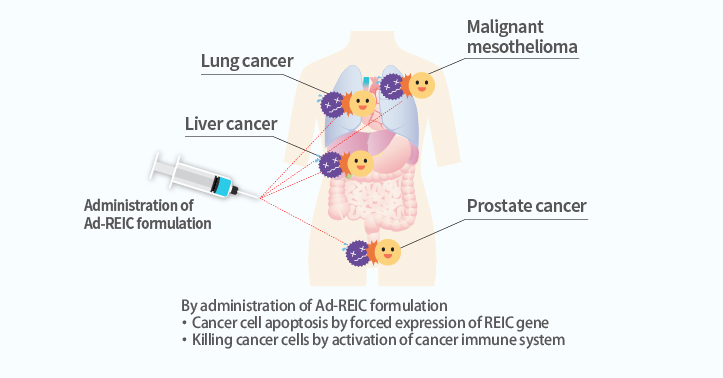
We are working on the development of "Next Generation Cancer Therapeutics" by gene therapy using Reduced Expression in Immortalized Cell (REIC) gene which was discovered at Okayama University.
Although the REIC gene is expressed vigorously in normal cells, its expression is reduced when cells turn into cancer cells. It is revealed that forced expression of the REIC gene in cancer cells causes selective cancer cell death (apoptosis) and cancer immunity activation.